Arabidopsis research
Arabidopsis and plant hormones
Plants are important for the earth’s ecosystems, as well as
human agricultures and civilization. Among all of the plants,
Arabidopsis thaliana (commonly known as thale cress or
mouse-ear cress) is often studied in science. Arabidopsis
are annual plants in the Brassicaceae family, which also
includes cabbage, broccoli, kale and radish. The small size
(20~25 cm tall), short generation time (~6 weeks) and high
seed yield (1000~3000 seeds per plant) make Arabidopsis the
ideal model plant to study in laboratories.
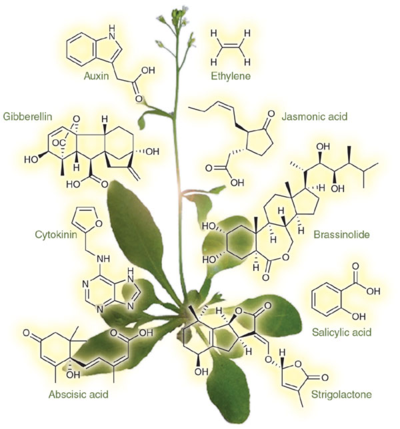
Similar to animals, plants depend on a number of hormones to
regulate growth. Hormones are small molecules made by
multicellular organisms that regulate the physiology or behavior
in another part of the body, and may be lipids, steroids or
peptides (small protein fragments).
Furthermore, recent advances of root-knot nematode infections
and gall formation have shown the importance of plant
hormones in parasitic nematode infections. For example,
pine wood nematode (Bursaphelenchus xylophilus) infections
promote the synthesis ofthe hormone ethylene, while beet
cyst eelworm (Heterodera schachtii) has been shown to utilize
the plant hormone cytokinin to form feeding cells. Furthermore,
plant defense hormones suchas salicyclic acid and jasmonic acid
and plant peptide hormones have been shown to regulate
root-knot nematode infections. Recently, root-knot nematodes
have been reported to utilize auxin to regulate gall formation.
We are working on further delineating how plant hormones
mediate the development of plant roots and affect root-knot
nematode infections.
Auxin
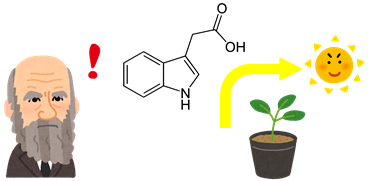
Our laboratory focus mainly on the hormone auxin and how it
regulates plant root growth. Auxin has been well-characterized
in the past, as it was first by Charles Darwin and continued to
be studied throughout the late 19th century. Auxin is mainly
known to stimulateplant growth, organ formation and
regulate responses to environmental cues. For example,
embryogenesis, vasculature formation, tropism and shade
avoidance are all known to be regulated by auxin.
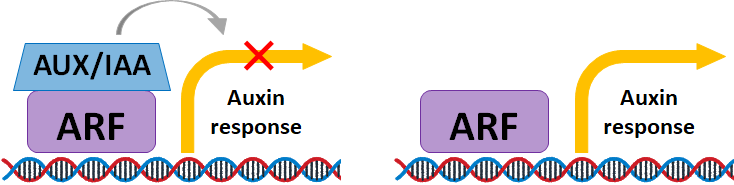
Many auxin-induced genes that regulate plant growth and
development have been characterized. Two particular
important proteins among these are the DNA-binding
protein AUXIN RESPONSE FACTOR (ARF) and the
transcription factor AUXIN INDOLE-3-ACETIC ACID
(AUX/IAA). The interactions between ARF and AUX/IAA is
important in regulating auxin-induced responses such as
embryogenesis and lateral root formation.
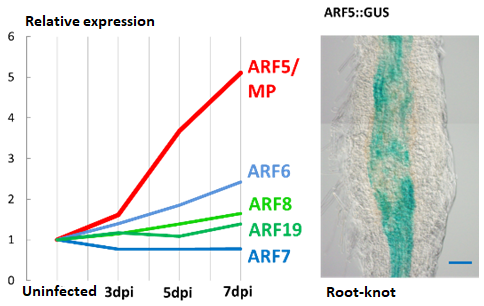
Our laboratory has conducted comprehensive analysis of
plant genes during root-knot nematode gall formation.
From our results, we found that the level of a particular
ARF protein, ARF5, increased substantially in developing
galls. Next, we found that gall formation rate was reduce
d in the mutant plant where ARF5 activity is abolished.
From these results, it’s clear that ARF5 mediates gall
formation through auxin signaling. Currently we
continue to decipher the role of ARF5 during gall
development.
Salicyclic acid
Plants synthesize and accumulate the defense hormone
salicyclic acid when challenged with pathogens.
However, these defense mechanisms are double-edged
swords as too much salicyclic acid can inhibit plant growth.
Therefore, plants must find the balance to optimize
growth and pathogen defense, although how plants
achieve this balance is not well-understood.
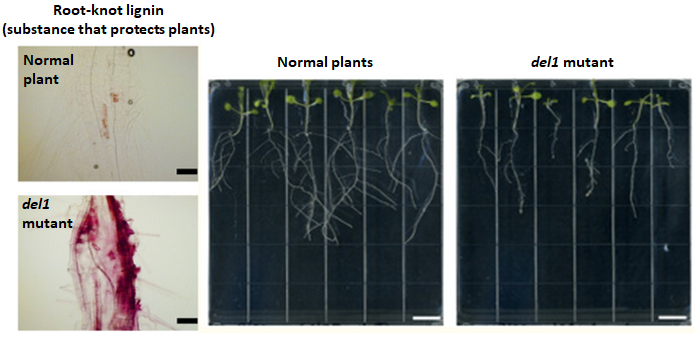
Our laboratory has identified the DEL1 gene as one such
regulator of growth-defense balance. The DEL1-deficient
mutant plants accumulate an excess amount of salicyclic
acid upon root-knot nematode infection, and show enhanced
resistance. On the other hand, these DEL1-deficient mutant
plants show reduced root growth even in the absence of
pathogens. Essentially, DEL1 maintains the plant’s growth-
defense balance through salicyclic acid level control.
Hopefully this DEL1-mediated balancing mechanism will be
able to contribute to the improvement of crop plants against
various pathogens.
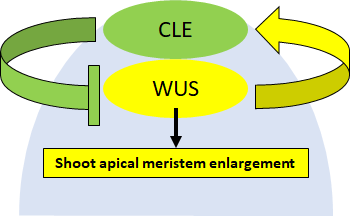
CLE peptide hormones
Aside from small molecules like auxin and salicyclic acid, plants also
synthesize peptide hormones consist of amino acids. These peptide
hormones are encoded in genes in the genome, which are transcribed
into RKN then translated into proteins in order to function. One of
the best-known plant peptide hormones is CLAVATA3 (CLV3) that
controls cell divisions in the shoot apical meristem (SAM), which
in turn forms all above-ground organs like leaves, flowers and fruits.
I
f CLV3 ceases to function, flowers make abnormal numbers of petals
and sepals while fruits become distorted. Even though the importance
of CLV3 in cell division is well-known, not all of CLV3’s functions are
fully-understood. Our research group have identified a signaling
component called heterotrimeric G proteins involved in CLV3 signaling.
We continue to discover new genes in peptide hormone signaling, and
delineate the mechanisms of how these genes regulate the appearances
of flowers, fruits and leaves.
The control of plant organ shapes is not only important for
above-ground organs, but also below-ground organs (i.e. roots)
as well. The roots provide mechanical support to the above-
ground organs, while also absorb water and nutrients from the soil.
Primitive ancestral plants actually have no roots, and roots are
a new feature that appeared during the evolution of more
advanced plants. As such, root formation actually utilizes many
mechanisms similar to those that regulate above-ground organs.
The root tip contains aroot meristems (RM), which is similar to
the SAM where cell division activities are regulated. Peptides
hormones similar to CLV3 called CLE peptides regulate cell
divisions in the RM. Our laboratory has identified several genes
that regulate below-ground CLE signaling to control RM cell
divisions such as BAM1, RPK2 and PUB4. With the understanding
of cell division regulation, it may be possible to decipher how plants
expand their roots in the soil. Furthermore, by comparing the cell
division regulatory mechanisms above- and below-ground, it may be
possible to understand how plants evolved by adapting signaling
pathways used above-ground in below-ground organs.
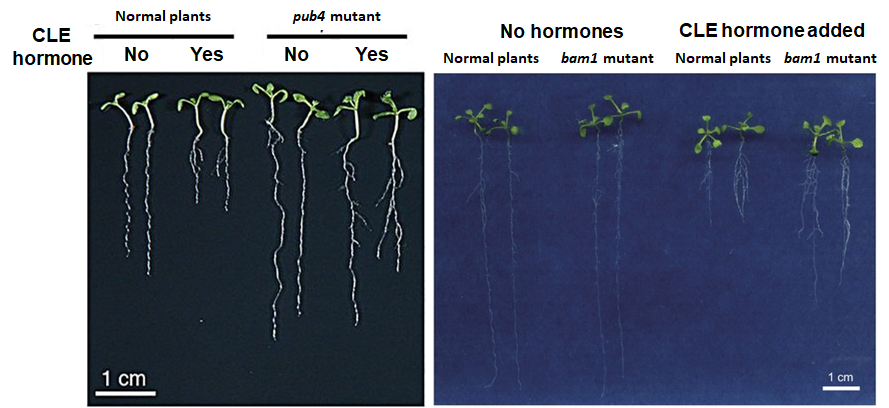
Image credits:
https://www.pathwayz.org/Tree/Plain/PLANT+HORMONES
Nakagami et al. (2020) Sci. Rep. 10(1): 8836.
Kinoshita et al. (2015) Development 142(3): 444-453.
Shimizu et al. (2015) New Phytol. 208(4): 1104-1113.
